Informationen über ElectroForce Cardiovascular Testinstrumente
ElectroForce Cardiovascular Testinstrumente
Bose GmbH
|
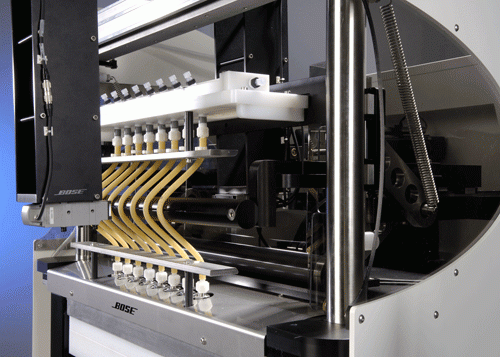
ElectroForce® stent/graft test (SGT) instruments have set the standard of performance for the fatigue testing of a variety of stents and endovascular grafts. Ten-year FDA in vitro tests take only weeks with ElectroForce instruments due to their reliable, accelerated dynamic performance. Specific SGT instrument designs are available for coronary and neural stents, abdominal stents, AAA bifurcations, and thoracic stents. For multi-mode peripheral and coronary stent fatigue testing, ElectroForce multiaxial test instruments provide realistic motion simulation to effectively reproduce in vivo conditions for multiple stents. The instruments are capable of testing an accelerated 10 year simulated life, performing fatigue to fracture studies, or evaluating worst case conditions for a stent design. For the fatigue and durability testing of coated and bioabsorbable stents, Bose has introduced the ElectroForce 9210 drug-eluting stent test instrument. This system provides a new standard of performance resulting in expedited time to market and improved testing accuracy. Through a combination of new and existing proprietary Bose technologies, the 9210 instrument allows a user to test twelve specimens simultaneously, provides increased distention uniformity along the stent profile and combines high-frequency performance with the necessary precision for particle capture. ElectroForce multi-specimen fatigue testing systems can be used for high cycle fatigue life characterization of coronary and vascular device structures, and evaluation of device materials for s/n curve development. The test systems provide displacement controlled loading for small soft structures and devices such as stents, stent segments, vena cava filters, septal closure structures and annuloplasty devices.
|





